| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
May 2, 2014 - A complex network of luminal structures plays an important role in bodily functions such as transporting gases and fluids associated with metabolism or homeostasis. The tube length and diameter of these tubular networks vary according to size of the animal, the location inside the body, and of the molecules that will circulate through the tube to ensure efficient transport. The Drosophila embryo, for example, prior to hatching, develops a tracheal tube that fits its tiny body. Past studies of this tracheal system indicate that apical membrane synthesis, lumen components, and cell-cell adhesion between epithelial cells have an important role in tracheal tube formation; however, the detailed mechanism has remained unclear. In a new study published in Cell Reports, Foreign Postdoctoral Researcher, Bo Dong, from the Laboratory for Morphogenetic Signaling (Shigeo Hayashi, Group Director) in collaboration with Edouard Hannezo of the Institut Curie in France reveal that the extracellular matrix (ECM) plays a regulatory role in determining tube length. Using the Drosophila tracheal system, they demonstrate that elastic ECM and its interaction with the encircling apical membrane of the tracheal epithelium are key factors in regulating tube length.
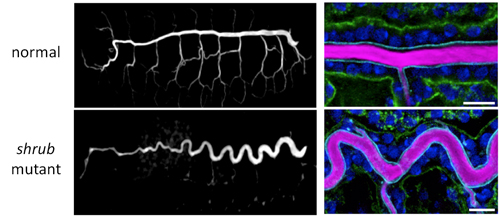
The Drosophila tracheal system consists of a thick tube of epithelial cells running through the center of the body with smaller and narrower tubes branching off to the dorsal and ventral sides. During development, the tracheal lumen is filled with ECM, which is broken down prior to hatching and replaced by gases allowing it to fill its physiological role. The tracheal epithelial cells are arranged with their apical membrane facing the lumen and the basal membrane facing the outside. Dong et al. first searched for mutants showing abnormalities in tracheal tube length, and came across a fly mutant that had an abnormally long and sinusoidal-shaped tracheal tube, but with a diameter similar to that in wild-type flies. This fly had a mutation in the shrub gene, which encodes part of a protein complex involved in membrane remodeling for vesicular transport. They examined the cell membrane on both apical and basal sides of trachea epithelial cells by immunostaining each side with different colored markers and found that, compared with wild-type, only the apical membrane of shrub mutants had elongated along the anterior-posterior (AP) axis. The basal membrane was nearly unchanged and there were no significant differences in cell number. The defective transport machinery in shrub mutants results in many membrane proteins accumulating in the cytoplasm rather than at the membrane; the apical membrane protein Crumbs (Crb), which is involved in apical membrane biogenesis, is one such protein. Crb is normally localized to the apical membrane, but in the shrub mutant, Crb was found amassed in the endosome, stimulating apical membrane overgrowth. As the orderly wave-like pattern of the trachea tube in shrub mutants suggests a mechanical effect, such as a force antagonistic to the elongational force of the apical membrane, the group shifted to focus on the ECM filling the tracheal lumen, which is made up of chitin, a polysaccharide that uniformly fills the lumen, and of protein components such as Dumpy (Dp) a giant protein involved in maintaining epithelial integrity. They used GFP-tagged luminal proteins and FRAP (fluorescence recovery after photobleaching) experiments to investigate the physical properties of Dp and found that while it barely moves within the lumen, the protein itself is stretched as the tube elongates. Thus, the resistance from the elastic ECM is likely the force counteracting the elongating force exerted by the apical membrane. Taking their findings, Dong et al. established a physical model of tube length that recreates the mechanism through computer simulation. Their model assumes that the apical membrane encircles and binds to the elastic cylindrical matrix; when apical membrane growth is stimulated, it creates an elongating force along the AP axis, which will in turn stretch the ECM. The trachea will stop elongating when the apical membrane’s elongating force and the ECM’s elastic force are balanced. The group was able to verify the relevance of their model by confirming that it gave close predictions of the actual phenotypes observed in shrub and other mutants with varying lengths and shapes of elongated trachea. “As one of the major components of the body, the ECM is often seen as having a supportive role in the body, but our current study reveals that it has a prominent role in regulating morphogenesis as well,” says Hayashi. “We hope to continue closing in on the mechanisms underlying how shapes of organisms, which are closely associated with function, are determined.” |
|||||
|
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |