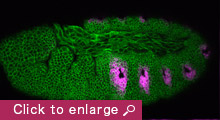
Our research aim is to understand fundamental mechanisms of animal morphogenesis with particular interest in the mechanical basis of tissue movement and its interaction with the extracellular environment. Our main research focus is the tracheal system in the Drosophila embryo, a network of tubular epithelium used as a respiratory organ. Trachea is formed through invagination, tube formation, elongation, fusion, and final maturation into a respiratory organ. We are particularly interested in the mechanical control of epithelial architectures. Epithelium is stabilized by cell-cell adhesion and cell-matrix adhesion. Breaking this stability is essential for initiating morphogenetic movement. We found that prospective tracheal primordium is under negative tension (pressurized). Anisotropic redistribution of tissue tension and timely mitosis initiates local mechanical instability that leads to tissue invagination movement (Kondo and Hayashi, 2013). Once the tracheal network is formed, tube diameter and length are enlarged to reach the final size. Tracheal size change involves increase in cell size, especially an increase in apical cell area facing the luminal side. A key question is how individually controlled cellular growth is coordinated to form coherent tissue architecture. We found that extracellular matrix in the luminal space plays a central role by providing mechanical stability to the tubules (Dong et al., 2013, 2014). Defects in extracellular matrix components lead to destabilization of tube shape and malformation, resulting in tubule morphology seen in organs under pathological conditions.
Another research area of interest is the mechanism of cell morphogenesis. Here we ask the question to what extent single cells can autonomously organize nanometer scale cellular patterns. Our studies have uncovered the role of the cellular trafficking center as an organizer of cell elongation (Otani et al., 2011).

A:…
A:…
A:…
A:…
A:…
A:…
A:…
A:…
shayashi[at]cdb.riken.jp