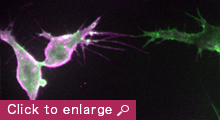
The establishment of an intricate network of interconnected blood vessels is essential for the development of many tissues and organs. Tissue vascularization frequently occurs through sprouting angiogenesis, where new blood vessels arise from pre-existing ones, and encompasses a multitude of cellular processes including polarized collective cell migration, proliferation, anastomosis and lumen formation. While many key molecules and signaling pathways have been identified to regulate endothelial tip/stalk cell specification, blood vessel guidance and arterial-venous differentiation, there is still a poor understanding of how angiogenic signals are relayed to the cell’s machinery to drive changes in endothelial cell morphology, behavior and consequently, the final pattern of the vasculature.
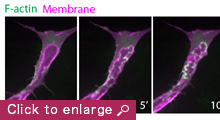
My laboratory aims to unravel fundamental mechanisms that regulate endothelial cell dynamics and coordination during blood vessel morphogenesis. Previous studies on actin cytoskeleton revealed that specialized F-actin of different dynamics and subcellular localization drive distinct steps of vessel morphogenesis. For example, the transient polymerization of F-actin at the apical membrane controls lumen expansion while a stable pool of F-actin at endothelial cell junctions stabilizes nascent lumens to produce a functional vascular network. In the future, we aim to investigate the role of force generation in regulating endothelial cell dynamics and vessel morphogenesis, the mechanisms controlling actomyosin activity in endothelial cells and the molecular regulation of vessel lumen formation. Our long-term goal is to understand how upstream angiogenic signals as well as hemodynamic forces regulate force generation and endothelial cell behavior.
To achieve our research goals, we employ live imaging at high temporal and spatial resolution, advanced fluorescent microscopy techniques, genetics and chemical biology to elucidate the mechanical and molecular mechanisms of blood vessel morphogenesis using the zebrafish as our model organism.

likun.phng[at]riken.jp